Revamping the Animal Drug User Fee Act Should Not Override Public Health
In speeding new animal antibiotics to market, Congress also must ensure better stewardship.

Coauthored by Sameer Patel, MD, MPH, a pediatrician and infectious disease specialist at Northwestern University’s Feinberg School of Medicine and director of the Antimicrobial Stewardship Program at Lurie Children’s Hospital in Chicago.
Dangerous infections caused by antibiotic-resistant bacteria known as “superbugs” are among the world’s biggest health threats. They kill more than a million people each year. By 2050, up to 10 million annual deaths are predicted.
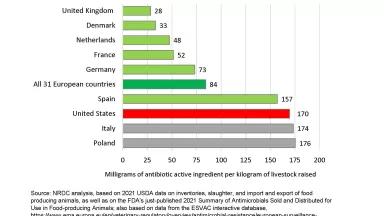
The anticipated surge in deaths is due to an increased prevalence of bacteria that are resistant to antibiotic drugs. The culprit is excessive antibiotic use—overuse—which is unnecessary and avoidable. For decades, infectious disease experts have warned that overuse speeds up the development and spread of more dangerous superbugs. And yet penicillins, tetracyclines, and other antibiotics continue to be overused on U.S. farms as well as in hospitals. A recent NRDC analysis shows that medically important antibiotics in 2020 were being used twice as intensively in U.S. pigs as in human medicine, and the pig rate of use is 12 percent higher than it was in 2017.
The solution is for federal agencies to implement a whole-of-government antibiotic stewardship program, starting with identifying and curbing overuse wherever it occurs. For its part, the Centers for Disease Control and Prevention (CDC) has beefed up efforts in recent years to closely track and respond to antibiotic overuse in human settings. The CDC sees these as critical steps for measuring and improving human antibiotic stewardship. By contrast, the U.S. Food and Drug Administration (FDA) has no plans or systems in place to track and identify overuses of these same antibiotics on pigs, cattle, and other food animals, nor to curb them. This year represents the fifth and final year of the FDA’s inaugural veterinary antibiotic stewardship plan. Ironically, that plan has never contained goals or indicators to measure the level of stewardship from year to year, or to verify that stewardship in 2023 is better or worse than it was in 2018. There is clear statutory authority for the FDA to do this kind of tracking and measuring. Through its actions, however, the FDA has demonstrated an unwillingness to exert that authority unless Congress directs it to do so.
Senate leaders must provide the FDA with explicit direction as part of its 2023 reauthorization of the Animal Drug User Fee Act (ADUFA). The current law, which funds a significant portion of the FDA’s budget for overseeing animal antibiotics, expires on September 30. The House of Representatives already approved a “reauthorized” or updated ADUFA bill in July. Like the expiring legislation it would replace, however, the House bill fails to require the FDA to measure and report on whether its stewardship activities have actually improved stewardship. Measurement is paramount if the FDA is to hold the animal agriculture sector accountable for doing its part to help address worsening resistance by finally reducing rates of antibiotic use, which mostly remain very high.
The Senate has yet to pass its own ADUFA bill. On September 6, 2023, therefore, 19 leading medical, infectious disease, animal welfare, pandemic preparedness, and environmental organizations sent a letter imploring Senate leaders to only pass a new ADUFA bill if it includes much-needed public health protections. The organizations, including the pair to which the authors belong, believe that without these protections U.S. efforts will fall short to combat the spread of increasingly dangerous superbugs, along with the antibiotic overuse that drives it.
The antibiotics being given to U.S. farm animals today were often FDA-approved years or decades ago, before the danger of overusing antibiotic was fully recognized. Yet, on the industrial farms typical of U.S. livestock production, these drugs continue to be given to flocks and herds of animals on a routine basis, hastening the spread of resistant bacteria into surrounding communities. Drug-resistant infections can take hold in people who handle these animals or eat contaminated food products derived from them, as well as among people coming into contact with drug-resistant bacteria in manure-contaminated soil, air, and water.
Congress first passed ADUFA legislation in 2003. Since then, the ADUFA has remained as industry-led legislation that is tightly focused only on getting new animal drugs, including antibiotics, more quickly approved by the FDA and into the marketplace. Unless Congress seizes the moment, the FDA will again deploy the ADUFA-generated user fees in ways that do nothing to address the overuse of existing antibiotics.
Our recommended changes to the ADUFA are long overdue. As physicians and public health advocates, we urge members of the Senate to move the needle on antibiotic stewardship in the updated version of the ADUFA they will soon vote to move into law.